Computer-aided detection (CAD) software developer EDDA Technology of Princeton, NJ, has received the European CE Mark for its liver MDCT package, the company said.
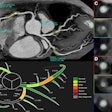
EDDA's IQQA-Liver software offers automated quantitative volumetric evaluation of CT data of the liver, liver lobes and segments, hepatic lesions, and vascular structures including arteries, portal veins, and hepatic veins. The package allows for fast evaluation of imaging data as well as operative planning simulation of tumor resection.
IQQA-Liver already has clearance by the U.S. Food and Drug Administration, as well as China's equivalent, the State Food and Drug Administration (SFDA). It is currently being used by clinicians at University of Colorado Hospitals Denver, Shanghai Zhongshan Hospital, Tianjin First Center Hospital, and Beijing You'an Hospital. Regulatory clearance of IQQA-Liver in Taiwan is pending, according to EDDA.
Related Reading
Shimadzu, EDDA partner on x-ray CAD, November 11, 2009
Road to RSNA, Advanced Visualization, EDDA Technology, November 6, 2008
Road to RSNA, CAD, EDDA Technology, October 30, 2008
EDDA launches new liver CAD version, July 24, 2008
Road to RSNA, CAD, EDDA Technology, November 1, 2007
Copyright © 2009 AuntMinnie.com