Portable scanner developer NeuroLogica has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its BodyTom CT scanner.
BodyTom, the newest member of NeuroLogica's CT line, is a 32-slice scanner with an 85-cm gantry and 60-cm field-of-view. The battery-powered system can be transported from room to room, similar to mobile x-ray systems, according to the company.
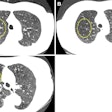
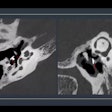
The scanner is compliant with the DICOM 3.1 standard, and it's capable of providing images in operating rooms, emergency departments, or intensive care units without having to move patients to the radiology department.