Virtual colonoscopy is a paradigm shift from traditional optical colonoscopy, and it needs to be used in a way that targets rare cancer-causing polyps, rather than each and every growth in the colon, according to David Kim, an associate professor at the University of Wisconsin.
"We have been using the polyp as the surrogate target for the true target lesion, but targeting the polyp is inefficient," he said. "The advantage is that it is most likely to remove the true precursor. The disadvantage is we remove many polyps of no consequence."
Kim pointed to the impact of unnecessary polypectomies: They increase costs and divert limited colonoscopic resources from screening efforts. But the biggest impact, according to his research, is on patients.
"[Universal polypectomy] is an easy answer but not a good one," Kim said. "The perforation rate is one in 1,000. One-third require resection. Seven percent die. A healthy person dies because they sought screening."
Kim advocates using virtual colonoscopy (also known as CT colonography or CTC) for screening with optical colonoscopy and polypectomies as a follow-up, if needed. He explained his rationale for this screening paradigm at the Society of Gastrointestinal Radiologists (SGR) meeting, held in Carlsbad, CA, in March.
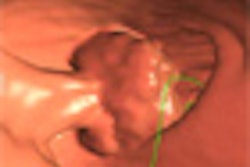
"The pathway to all cancer is increasing dysplasia over time," Kim said. "The target is adenoma that harbors high-grade dysplasia."
Polyps that are 10 mm or larger, or finding more than two polyps larger than 6 mm, should prompt a polypectomy, Kim said. Finding one or two polyps between 6 and 9 mm in size should prompt either polypectomy or surveillance for up to three years, depending on the patient and the polyp.
Smaller polyps should go unreported, he said.
"We put people in a routine five-year follow-up," Kim said. "This approach we use in radiology every day -- we do it in a pulmonary nodule, for example, right?"
Kim said an important component of the strategy is to require VC every five years, where postcolonoscopy patients aren't expected back for 10 years.
"That [10-year] follow-up isn't based on any particular study," he said, pointing out that colorectal cancer is particularly slow to grow. "There are some high-risk targets, but if you check every five years, you'll catch them."
"Some people equate every soft-tissue polyp with cancer, and that is certainly not the case," Kim said. "It's really important to understand that the majority of adenomas do not become cancer. What we are really looking for is a tiny subset of soft-tissue polyps."
Kim sorted through the types of growths that are typically removed and focused on the two small subsets that become cancer: a subset of hyperplastic polyp called a serrated polyp and advanced adenomas, both a relatively small portion of the number of polyps found.
"It's really important to understand the majority are defined by their large size," he said, estimating that 90% of the malignant growths can be detected by size larger than 10 mm. "They also share the trait of high-grade dysplasia," Kim said.
For many, the idea of leaving smaller polyps behind, with the accompanying risk of cancer in the long term, is an unpalatable approach. But Kim argues that the risk is minimal.
"Existing data suggest the risk is manageable," he said, citing a study by Lieberman and colleagues in Gastroenterology (October 2008, Vol. 135:4, pp. 1100-1105).
In 2007, Kim led a study that looked at outcomes for 3,120 patients who underwent VC compared to 3,163 who had traditional colonoscopy. While the detection rates of advanced adenomas and advanced neoplasia were about the same, four times the number of polyps removed after VC (561) were removed during a standard colonoscopy -- a total of 2,434 polyps. Seven of the colonoscopy patients suffered complications, while none of the VC patients did.
"You can make the argument for polypectomy in optical colonoscopy because the patient is already there, already prepped," Kim said. "That's why the universal polypectomy is so common. But it is not necessary."