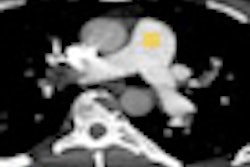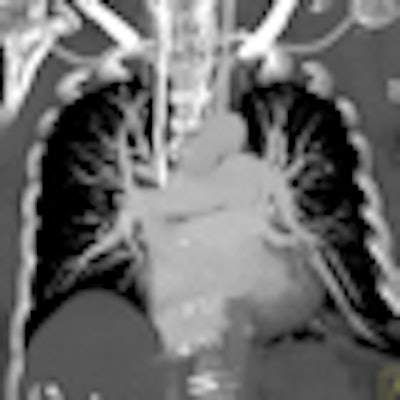
Perfusion defect size on dual-energy CT of pulmonary embolism (PE) is an independent predictor of both right heart strain and patient outcomes, concludes a new study by German and U.S. researchers. On the other hand, lab markers such as D-dimer results showed only weak correlations.
"We found that the relative perfusion defect size was actually the strongest predictor for patient outcomes in this study, followed by right heart strain," said Dr. Ralf Bauer from Goethe University in Frankfurt am Main in a presentation at the 2011 European Congress of Radiology (ECR).
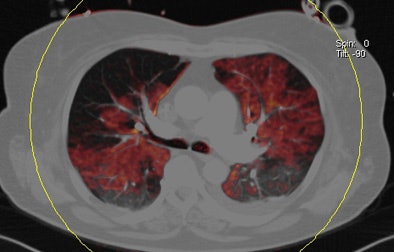
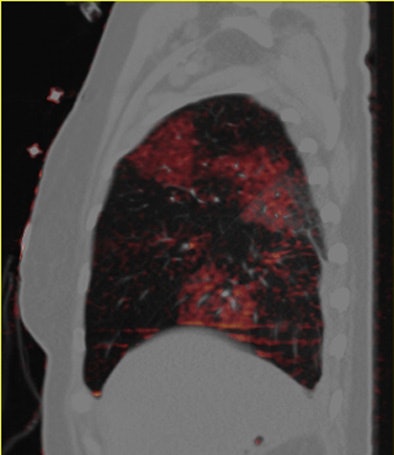
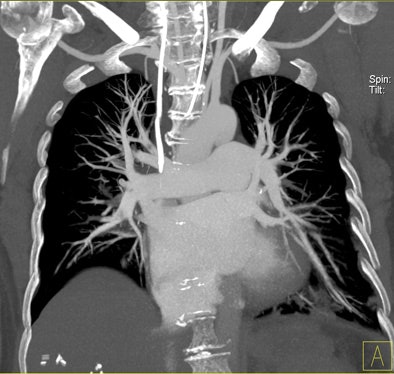
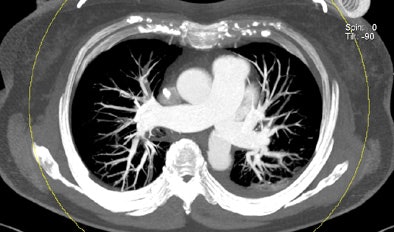
Dual-energy CT enables the selective display of iodine distribution within the pulmonary parenchyma following contrast-enhanced pulmonary CT angiography, which in turn permits the calculation of perfusion defect (PD) size in patients with suspected PE.
Depending on the shape and pattern of the defects at dual-energy CT, perfusion defects due to PE can generally be distinguished from, for example, patchy defects signaling other pathologies of the lung parenchyma, Bauer said.
But while defects can readily be measured, determining their significance to the patient's clinical condition is another matter, he said.
"We can display the perfusion defects, but so far there's no data available on what it means to us," Bauer said.
Is it possible to see correlations between perfusion defect size and surrogate markers of PE severity, such as laboratory markers or right heart strain? Does PD size and the presence of heart strain affect survival?
Bauer and his team from Goethe, along with Dr. Joseph Schoepf and colleagues from the Medical University of South Carolina, aimed to find out in their retrospective analysis of data from 53 patients with acute PE who underwent dual-energy CT (Somatom Definition Flash, Siemens Healthcare).
The researchers measured perfusion defect size in the lung parenchyma caused by PE on dual-energy iodine distribution maps, and expressed it in two ways: as absolute quantification (VolPD in mL) and relative to the total lung volume (RelPD in %).
Signs of right heart strain were determined using multiplanar reconstructions in a cardiac four-chamber view. Patient records were searched for D-dimer, pO2, and pCO2 levels at admission; echocardiography signs of right heart strain; and information on readmission for recurrent onset of PE and death.
The limited field-of-view on dual-energy scanners was a significant drawback for dual-energy CT, Bauer noted.
"When we did lung perfusion, the analysis was restricted to the field-of-view," which is limited due to the smaller second detector on the dual-source CT scanner, he said. As a result "there were 40 patients with no coverage loss, eight patients with up to 5% coverage loss, and five patients with up to about 10% of coverage loss in the peripheral" areas of lung parenchyma, he said.
Seventeen patients showed evidence of right heart strain on CT, which was consistent in every case with the echo findings, Bauer said.
"We found a fairly large variety of perfusion defect sizes," he said.
VolPD ranged from 5.8 mL to 748.4 mL, and RelPD ranged from 0.2% to 22.9%.
There was no correlation of perfusion defect size with blood gases pO2 and pCO2, and PD size was only weakly correlated with D-dimer levels (r = 0.43-0.47; p < 0.003).
Of course, D-dimer "isn't very specific and can be increased in various conditions like tumors and after surgery," Bauer said.
The 17 patients with right heart strain had significantly higher perfusion volume (215.4 mL versus 73.3 mL, p < 0.01) and relative perfusion defect sizes (9.9% versus 2.9%) compared to patients without right heart strain (perfusion defect sizes for both perfusion defect volume and relative perfusion defect size), Bauer said.
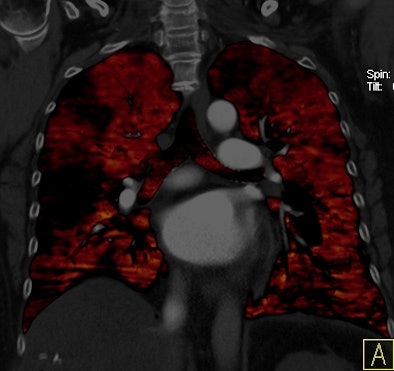 |
| Patient with bilateral segmental and subsegmental PE and large perfusion defects on dual-energy iodine distribution maps. All images courtesy of Dr. Ralf Bauer. |
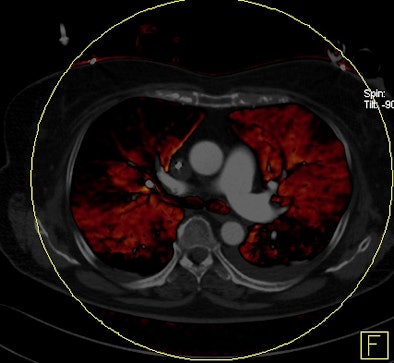 |
 |
 |
 |
 |
A subpopulation analysis of patients with PD volumes greater than 5% or relative PD volumes greater than 2.5% showed that both deaths and the number of readmissions for PE were higher in patients with relative perfusion defect volumes greater than 5% -- and there was no correlation for other patient groups, Bauer said.
Key to the results, survival was significantly reduced for the group with relative perfusion defects greater than 5% (n = 17) compared to those with 2.5% to 5% (n = 16) or less than 2.5% (n = 19) relative PD sizes, who showed no hard PE end points.
In addition, the relative hazard ratio for death was increased between 3.7 and 10 for the group with more than 5% relative PD size, Bauer said.
The group with greater than 5% relative PD experienced two deaths and one readmission due to PE.
Mean survival time versus relative volume of perfusion defects (RelPD)
|
The small sample size was the most important limitation of the study, Bauer said. In addition, "it was a heterogeneous patient cohort in terms of underlying risk factors," he said. "We had many oncologic patients, patients after surgery, and patients after long-distance flights," he said. Nine patients were lost to follow-up, and the limited field-of-view of DECT scanners "may be a confounding factor for the reliability of our results."
The study not only confirms the results of Chae et al and Zhang et al, who found a strong association between right heart strain with perfusion defect size, it establishes that relative perfusion volume is an independent predictor of patient outcomes, Bauer said.
"Relative perfusion defect size is actually a stronger predictor than right heart strain in this study population," he said. "A high relative perfusion volume defect will go along with a high risk of death, but of course we need a prospective trial setting to confirm it."