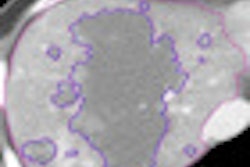
GE Healthcare has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Veo CT dose reduction technology.
The model-based iterative reconstruction (MBIR) technique is designed to produce diagnostic-quality CT images with less radiation dose. Veo is currently available on GE Discovery CT750 HD systems in Europe, Canada, and regions of Asia.
GE is also coordinating a multicenter study to investigate further improvements using Veo, including even lower dose levels, across a range of applications.