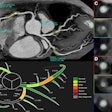
Siemens Healthcare has received U.S. Food and Drug Administration (FDA) clearance for its Somatom Definition Flash dual-source CT scanner equipped with the vendor's new Stellar detector.
Designed to virtually eliminate electronic noise, the integrated detector yields high spatial resolution, ultrathin slices, and image sharpness at low patient radiation levels, according to the vendor.
Stellar employs Siemens' TrueSignal technology, which integrates electronic detector components into the photodiode to reduce power consumption. The result is a significant reduction in electronic noise and improvements in signal-to-noise ratio, Siemens said. Stellar also utilizes Siemens' Ultra Fast Ceramics detector material.
Thanks to Stellar and its maximum temporal resolution of 75 ms, Definition Flash can now perform cardiac scanning in a quarter of a heartbeat, Siemens said. Cardiac motion can be frozen, allowing clinicians the option of not requiring patients to hold their breath during the exam, according to Siemens.
Siemens has also included its FAST CARE (Fully Assisting Scanner Technologies/Combined Applications to Reduce Exposure) workflow acceleration and dose reduction technology.