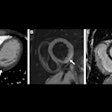
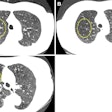
Siemens Healthcare has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Somatom Definition Edge single-source CT scanner.
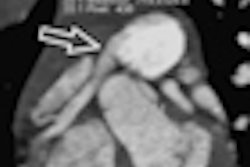
Definition Edge is the first single-source CT scanner to use the vendor's Stellar Detector, which integrates the detector's miniaturized electronics with the photodiode, Siemens said. The scanner can visualize structures up to 0.30 mm, with potential improvement in image sharpness, according to the vendor.
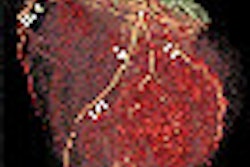
Definition Edge is the company's fastest single-source CT scanner, offering a rotation speed of up to 0.28 sec. Its new dual-energy mode allows the system to scan at two different tube voltages to help characterize tissue types, Siemens said.
It has a temporal resolution of 142 msec and allows for a pitch of 1.7, which enables an acquisition speed of up to 23 cm/sec, Siemens said. The system is also capable of reconstructing 384 slices per rotation. Definition Edge will begin shipping in the summer.