CT nanoparticle-based contrast agents are all investigational -- and at this point all preclinical -- but they're out there. In a few years, the tiny contrast agent delivery vehicles, which work well in animals, could greatly affect the diagnosis and treatment of disease in humans.
That's according to David Cormode, PhD, assistant professor of radiology at the University of Pennsylvania. He spoke on nanoparticle CT contrast media at last month's International Society for Computed Tomography (ISCT) annual meeting in San Francisco.
The integration of nanoparticle CT contrast into the clinical mainstream will take a while, Cormode said, but the process might be speeded up if elements are developed that are cheaper than gold, which is currently used to make the nanoparticles.
Nanoparticles for CT imaging aren't so different in design and structure from those built for other imaging modalities such as MRI, except that they contain CT contrast agents in their lipid-based cores. Inside every nanoparticle agent is an inner core surrounded by various layered and scattered components including liposomes, micelles, emulsions, and nanocrystals with biocompatible coatings, Cormode said.
CT contrast agents have bigger cores than, for example MR agents, because they need them. Due to CT's relative insensitivity to contrast compared to other imaging modalities, high-contrast payloads are required for better sensitivity, Cormode said. Nevertheless, they are still quite small, typically less than 4 nm in diameter.
"If you compare the Earth to the size of a soccer ball, it's about 58 millionth of the size," he said. "Going from [the soccer ball] to a nanoparticle, it's about the same factor of difference in sizes."
In recent years, interest in imaging nanoparticles has soared, with about 80 peer-reviewed studies being published a year, compared with fewer than 10 as recently as 2006, Cormode said. The reason is, quite simply, their vast potential in diagnosing and treating disease.
Nanoparticles are designed to be long-lasting contrast agents that do not need to be readministered in the case of multiple exams being acquired over several hours or potentially even days, Cormode said.
"Compared to agents we are currently using, nanoparticles have long circulation half-lives," he said. "They can be targeted to allow molecular imaging or specific cells or specific processes. They can be used with spectral CT, and they can also be multifunctional, providing contrast for more than one imaging technique."
Although no nanoparticle agents are approved for CT, a couple have been cleared for use with MRI, including Doxil, a liposomal formulation of doxorubicin approved for head and neck cancers in the mid-1990s, and Feridex, an iron-oxide nanoparticle contrast agent.
The tiny structures have become more complex in recent years, featuring multiple layers of different coatings, and an antibody or protein used to direct the agent to a biological target. For example, a 2009 in vitro and rat study by Pan and colleagues examined an iodine-loaded agent targeted to fibrin for thrombus imaging, he said.
Representing a new class of nanoparticles designed for CT, the colloidal, radio-opaque and metal-encapsulated polymeric (cROMP) particle offered severalfold enhancement both in vivo and in a rat model, its authors reported, with sensitivity reaching to the low nanomolar particulate range (Journal of the American Chemical Society, October 2009, Vol. 131:42, pp. 15522-15527).
Key CT nanoparticle studies
In 2006, Mukundan and colleagues tested a blood-pool contrast nanoparticle encapsulating a high concentration of iodine. They injected the agent into five mice and scanned them with micro-CT. The researchers measured high initial enhancement of about 900 HU in the aorta, which plateaued at about 800 HU when measured again two hours later, and there was excellent contrast discrimination between the myocardium and cardiac blood pool (American Journal of Roentgenology, February 2006, Vol. 186:2, pp. 300-307).
"I imagine if they had continued the study for a longer time, you would have seen the same amount of contrast," Cormode said.
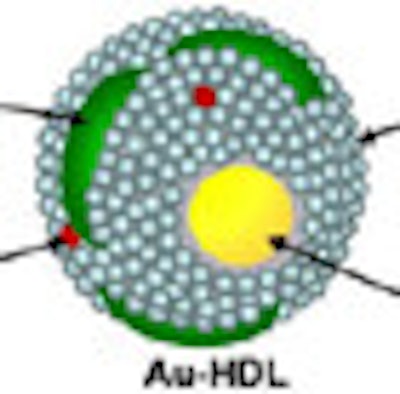
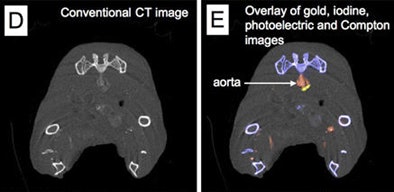
In a 2010 study of a gold high-density lipoprotein nanoparticle targeted at atherosclerosis, Cormode and colleagues showed that nanoparticles can distinguish several different components in a single scan. The group performed spectral imaging on a preclinical CT scanner developed by Philips Healthcare to differentiate gold (Au-HDL), iodine-based contrast, and calcium phosphate in phantoms. The gold nanoparticles were injected in mice and followed 24 hours later by an iodine-based contrast agent.
The gold particles were detected in the aortas of the mice, while the iodine-based contrast agent was highlighted in the blood and the calcium-rich tissue of the skeleton during a single CT scan. Microscopy showed that the gold was primarily localized in macrophages in the aorta, thereby showing that spectral CT also provided information about the macrophage burden (Radiology, September 2010, Vol. 256:3 pp. 784-782).
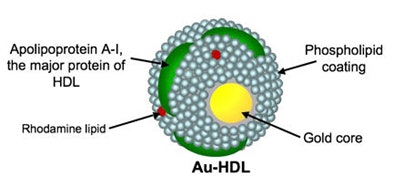 |
| Above, schematic illustration of macrophage-targeted gold core nanoparticle Au-HDL. HDL = high-density lipoprotein. Below, conventional CT image near bifurcation of aorta in mouse (left) contrasts with spectral CT images (right) following injection with Au-HDL and an iodinated emulsion contrast agent (Fenestra VC). Images republished with permission of Radiology, September 2010, Vol. 256:3, pp. 784-782). |
 |
"You can use nanoparticles ... to extract several different parameters at the same time," Cormode said of the study.
Also in 2010, Cormode collaborated with van Schooneveld et al to examine an all-in-one contrast agent for MRI, CT, and fluorescence imaging. The researchers created a gold/silica nanoparticle agent to enhance macrophage cells in vitro using MRI, CT, and fluoroscopy and mice livers in vivo using MRI and CT. The agent is useful in many applications, including cell tracking and target-specific molecular imaging, and is "a step in the direction of truly multimodal imaging," the authors wrote (Contrast Media and Molecular Imaging, July-August 2010, Vol. 5:4, pp. 231-236).
Diagnostic and therapeutic
Gold nanoparticle-based contrast was also the agent of choice in a study by von Maltzahn et al, who used a gold nanorod agent to visualize and then ablate tumors in mice. The nanomaterials improved the specificity of cancer ablation by homing into tumors and acting as antennas for externally applied radiofrequency ablation, the authors wrote (Cancer Research, May 1, 2009, Vol. 69:9, pp. 3892-3900).
The polyethylene glycol (PEG)-protected gold nanorods "actually absorb laser radiation very strongly, and when they do they heat up the surrounding material," Cormode said of the study. "This can be used as a kind of hyperthermia technique" to completely eradicate the tumors, he noted.
In summary, CT nanoparticle contrast agents "are out there, but gold is sort of expensive and we need to create cheaper agents," Cormode said. In addition, "extensive clearance and toxicity testing" will be needed before the agents are ready for routine clinical use.