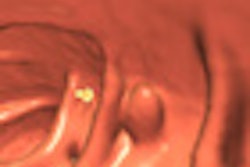
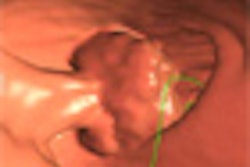
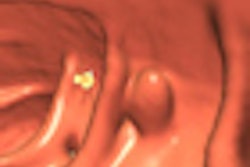
The U.S. Food and Drug Administration (FDA) has approved a new colon-cleansing product for use prior to colonoscopy.
Called Prepopik from Ferring Pharmaceuticals, the product is the first new colon-cleansing option approved in years. Prepopik (sodium picosulfate, magnesium oxide, and citric acid) was effective in multiple regimens in two separate studies involving a total of 1,200 patients scheduled for conventional optical colonoscopy, the agency said in a statement on Tuesday.
Prepopik consists of two packets of powder, each dissolved in cold water and taken at separate times. The preference is for a split-dose regimen beginning the day before the exam, but a same-day single-dose regimen also was effective, the agency said.
Individuals using the formulation must consume additional fluids before and after use of the agent, the FDA cautioned in its statement.
While the approval was limited to conventional optical colonoscopy applications, investigators will presumably begin testing the agent in virtual colonoscopy (also known as CT colonography or CTC) as well. As a condition of approval, the manufacturer agreed to conduct studies on the agent's safety in children.