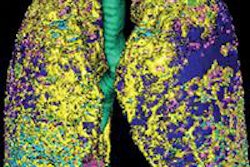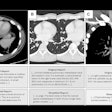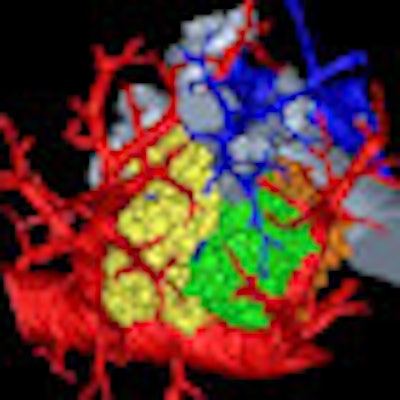
A research group has combined microCT with image processing tools to create a 3D rendering of the pulmonary acinus, which could enhance understanding of lung diseases. The model could also help clarify the role of acini in drug delivery, as sites where gases and blood mix in the lung.
The 3D model, which reveals the length, direction, and angles of the respiratory branches that lead to alveoli, allows the assessment of branch morphometry at the acinar level, according to the group led by senior author Eric Hoffman, PhD, a professor of radiology, medicine, and biomedical engineering at the University of Iowa.
"Advanced technologies are now allowing us to image the actual structure of the most peripheral respiratory units of the lung," Hoffman told AuntMinnie.com. "By such imaging, we can now better understand how to model the deposition of inhaled particles within the lung, providing potential for new insights into the lung's response to inhaled pollutants or to model new delivery systems for inhaled drugs. The methodologies can now also be applied to excised portions of lung from patients with lung disease to better understand the peripheral disease processes."
While the model was derived from mice, the team believes it can be easily adapted for human imaging. The group included first author Dragos Vasilescu, PhD, now a postdoctoral research fellow at the University of British Columbia. Vasilescu based his thesis on the research while at the University of Iowa. Their results were published online earlier this month in the Proceedings of the National Academy of Sciences.
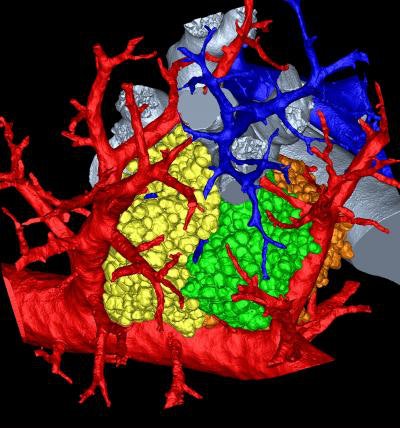 |
| 3D rendering of mouse pulmonary acini. Image courtesy of Dragos Vasilescu, PhD; the University of Iowa; and the University of British Columbia. |
Increasing disease understanding
With recent improvements in microCT imaging, the researchers became interested in applying imaging and image processing approaches to the mouse lung, hoping to take advantage of genetically altered mice to better understand disease processes to the level of individual alveoli, or air sacs, Hoffman said.
The Iowa researchers teamed with lung pathology experts Dr. Ewald Weibel, from the University of Bern in Switzerland, and Dr. Matthias Ochs, from Hannover Medical School in Germany, on the project.
"We have used our expertise in advanced CT imaging together with Drs. Weibel and Och's expertise to establish the methodologies for fixing the mouse lung in such a way as to preserve the in vivo structure, keeping the air spaces air filled," Hoffman said. "[This allowed] us to use some unique approaches to x-ray imaging to link microCT images of the in vivo lung to the ex vivo scans, such that we are able to establish the link between structure and function."
Image processing protocol
The image processing protocol takes the sampling methodologies and stereological assessment tools originally developed for use with microscopy and applies them to CT images. Interior scans of the parenchyma were performed at a resolution of 2 µm, which enabled the reconstruction and quantitative study of whole acini via image analysis and stereologic methods, according to the authors.
Because they imaged the lung in vivo and then ex vivo, the researchers were able to link the same locations of the lung both in vivo and ex vivo.
"By then using sampling principles established to help the microscopist subsample the lung in such a way that the subsamples were representative of the whole lung, we were able to direct the ultrahigh-resolution microCT scanner to focus in on specific areas of the lung and to provide 3D images of local acini, such that the small subset of reconstructed acini are representative of the whole lung, allowing us to make inferences regarding number and sizes of the peripheral lung components," he said.
The 3D reconstructions compared well with the architecture of silicon rubber casts of mouse acini, according to the researchers.
Working with 22 pulmonary acini culled from young and old mice, the team reconstructed the acini based on microCT imaging of scanned lungs both in the mice and also lungs extracted from them. These extracted lungs were preserved to keep the anatomy intact, and the researchers were able to use their technique to measure an acinus, estimate the number of acini for each mouse lung, and count the alveoli and measure their surface area, according to the researchers.
There are some limitations with the technique that still need to be overcome, including the long scanning times that are required to get such fine resolution, Hoffman said.
"With improved detectors, new methods of reconstructing the images with reduced noise, and solutions to eliminate other imaging-based artifacts, we will be able to obtain images faster and with less need for complicated image processing methodologies," he said. "By shortening the scan times and automating imaging and image analysis, the methodology can be applied to a larger number of lungs."
Human lungs
The researchers would now like to apply their methodology to human lung samples with the goal of answering some very basic questions regarding disease processes, Hoffman said. For example, recent research from Dr. James Hogg at the University of British Columbia found that the earliest process in the development of smoking-associated emphysema involves a loss of peripheral airways before alveolar destruction.
"The imaging methodologies we present can help to further explore this finding," Hoffman said. "Such a finding could lead to a better understanding of the root cause of the disease process."
In the next phase of the researchers' work, they plan to apply their methodology to mouse models of lung disease as well as human lung samples.
The current research received funding from the U.S. National Institutes of Health, the U.S. National Science Foundation, and the Swiss National Science Foundation.