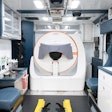
Chinese medical equipment manufacturer Neusoft Medical Systems has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its NeuViz 64 multislice CT scanner, the company announced.
The NeuViz 64-slice scanner delivers low-dose scanning, high patient throughput, and ease of use. It performs advanced cardiac imaging, and provides for a wide variety of clinically relevant postprocessing and diagnostic techniques, according to Neusoft.