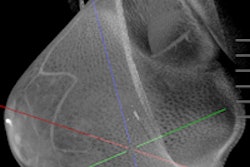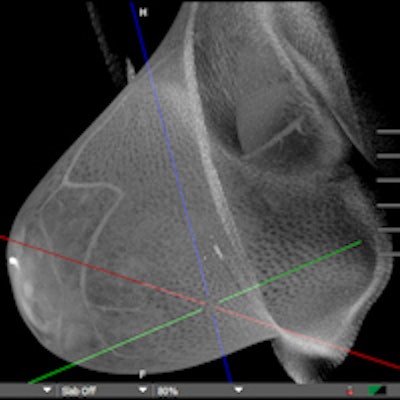
With approval from the U.S. Food and Drug Administration (FDA) now in hand for its breast CT scanner, Koning plans to market the 3D system as the ultimate one-stop diagnostic workup tool. Breast screening applications will have to wait, however, pending further study.
Koning Breast CT (KBCT) is the first commercially available 3D breast CT scanner designed specifically to image the entire breast with a single scan without compression of the breast tissue, according to the firm. Koning has high hopes for the unit, primarily due to its ability to overcome the tissue superimposition problem that vexes conventional 2D mammography.
"It completely eliminates any superimposition of tissue that you get with mammography and even tomosynthesis," John Neugebauer, Koning's senior vice president of sales and marketing, told AuntMinnie.com. "It can look behind implants and determine whether there are calcifications there or if the implant is compromised."
An adjunct to mammography
KBCT consists of a horizontal CT gantry and a patient table on which the woman lies prone, with the breast positioned in the imaging area. The product's FDA labeling calls for its use as an adjunct to standard two-view mammography as a diagnostic tool rather than as a screening device.
 The Koning Breast CT (KBCT) system. Al images courtesy of Koning.
The Koning Breast CT (KBCT) system. Al images courtesy of Koning.Exposure time for a single exam is 10 seconds; standard scan acquisition is 300 pulsed projection images, for a frame rate of 30 frames per second. The device may also be used with a biopsy bracket that allows for CT-guided breast biopsies, as well as a collimator that can be used to limit the x-ray beam to a particular area of interest, reducing radiation dose to the rest of the breast.
In fact, KBCT offers reduced radiation exposure compared to mammography, as only one follow-up KBCT study (at a maximum dose of 16 mGy) can replace multiple diagnostic mammography views (which can impart up to 32 mGy, depending on how many are performed), Neugebauer said. In addition, KBCT's radiation dose for biopsy procedures is about half that of stereotactic biopsy.
A long road
Koning has been pursuing approval for the device in the U.S. since 2008, when it filed a 510(k) application. But after three years, the FDA informed the company that it would instead have to file a premarket approval (PMA) application, which Koning submitted in 2012. The unit was cleared for sale in Canada and Australia last year.
"We met with the FDA, and they advised us that we had to conduct a similar study as had been done for digital breast tomosynthesis: a multireader, multisite study that compared the technology to diagnostic mammography," Neugebauer said.
More than 680 patient scans were conducted with KBCT at Elizabeth Wende Breast Care and the University of Rochester Medical Center, both in Rochester, NY, with additional collaboration at the University of Massachusetts Medical Center in Worcester, MA. Work conducted at these three sites culminated in a large reader study by Dr. Etta Pisano at the Medical University of South Carolina (MUSC).
Koning's clinical data showed that digital mammography plus KBCT produced an increase in sensitivity when compared with digital mammography alone, particularly with lesions smaller than 12 mm. Specificity of the two exams combined was slightly lower than digital mammography alone, however.
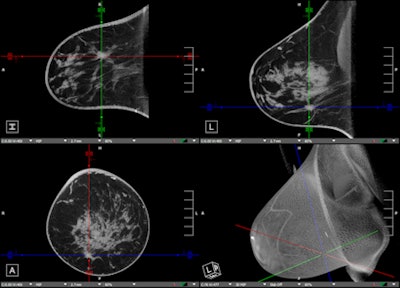 Breast CT: transverse, sagittal, coronal, and 3D views.
Breast CT: transverse, sagittal, coronal, and 3D views.Will breast imagers require extra training to use the device? Probably not, Neugebauer said.
"Reading these CTs is a lot easier than reading a mammogram, since you can stack the images and run through them in slices, or window and level them," he said. "The average radiologist has no problem stepping up to a CT scanner and interpreting the images, so we don't expect breast radiologists to require a lot of training. And the device's hanging protocols are the same as for mammography."
When to market?
Koning has been in discussions with potential partners, but for the time being the company will use its own sales force as it begins to put marketing efforts in place, Neugebauer said. Currently, there are four units installed: one at Emory University, one at University of Rochester Medical Center, one at University of Massachusetts Medical Center, and one at MUSC.
"These sites aren't ready to convert to commercial use yet," he said. "We aim to install another 10 units around the country before we really begin to market this. And, of course, the reimbursement issue needs to be addressed."
Going forward, Koning is investigating how to measure breast density with KBCT, using the brightness of lesions as measured by the Hounsfield unit scale as a possible predictor of malignancy, and whether the device can be used as a screening tool, Neugebauer said.
"We're prototyping a screening device and experimenting with another kind of detector that will get the image quality and dose to the levels necessary," he told AuntMinnie.com.