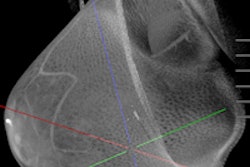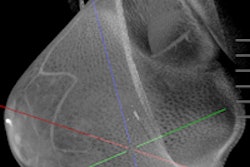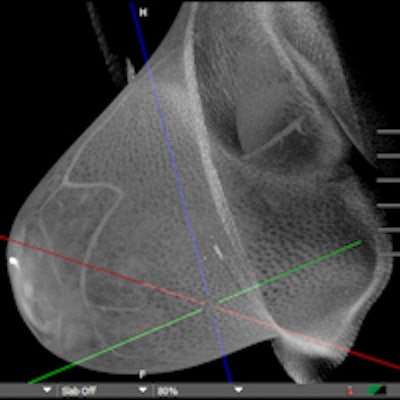
Koning on November 1 launched a screening trial for its Koning Breast CT 3D imaging device. It plans to use data from the trial to apply for clearance from the U.S. Food and Drug Administration (FDA) to market the scanner for breast screening.
The company received clearance from the FDA in 2017 to market Koning Breast CT for diagnostic mammography use. The system does not require breast compression.
The trial will be conducted at an imaging center operated by Radiology Imaging Associates in Port Orange, FL. Koning plans to submit its application to the FDA in the first quarter of next year.