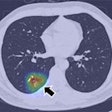
The U.S. Food and Drug Administration (FDA) is reporting class II recalls by Philips Healthcare of CT scanners manufactured by the company.
On July 24, the FDA announced that Philips has been recalling six models of CT scanners and one model of PET/CT scanner manufactured at its facility in Cleveland. Philips notified its customers of the scanner recalls in February 2015.
Philips said it was performing internal testing for a new software release for the scanners when it became aware of an issue that "could pose a risk for patients or users," if it were to reoccur, according to Mario Fante, senior group press officer for the company. The company analyzed its internal complaints and found that no incidents of harm from the issue have been reported. A probability analysis likewise found that "the worst of the failures are not expected to occur," Fante said.
Fante estimated that some 480 CT scanners were affected by the recalls. Philips field service engineers are in the process of upgrading the scanners with the firm's V4.1.3 and V4.1.4 software releases, which fixes the problem. A majority of affected scanners have received the upgrade, Fante noted.
Scanners affected by the recalls include Philips Brilliance CT 64-channel with Essence technology, Brilliance iCT, Brilliance iCT SP, Ingenuity Core, Ingenuity Core 128, and Ingenuity CT.
In a separate action that took place in June, the FDA reported recalls of three models of head-holder CT accessories. The head-holder accessories were recalled because they were released for use with other scanners but not yet certified for the Ingenuity, Ingenuity Core, and Ingenuity Core 128 systems.