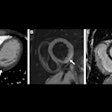
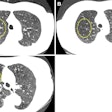
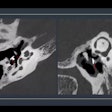
A colon cancer awareness group is criticizing this week's decision by the U.S. Preventive Services Task Force (USPSTF) to withhold its endorsement of two colon screening exams -- including CT colonography (CTC).
On October 6, USPSTF released draft recommendations on colon screening exams that largely reiterated existing guidelines. The proposed guidelines left out in the cold both CTC and the Cologuard fecal immunochemical DNA (FIT-DNA) stool test.
In an October 9 statement, the Colon Cancer Alliance said that USPSTF's stance will create barriers to the adoption of "proven screening methods for individuals unable or unwilling to have a colonoscopy." As a result, screening rates for colon cancer are likely to remain stagnant.
The group noted that USPSTF endorsed only the traditional stool-based FIT test, which has never been approved by the U.S. Food and Drug Administration but is considered "cleared" based on its ability to detect blood, rather than its ability to detect cancer. USPSTF acknowledged that the FIT-DNA test is better at finding cancer than the traditional FIT test, but the group was apparently worried about Cologuard's higher likelihood of generating false positives.
With respect to CTC, the Colon Cancer Alliance noted that the test is considered an acceptable alternative to traditional colonoscopy by many healthcare providers, and even President Barack Obama received a CTC exam to avoid unnecessary sedation.
"CT colonography is recommended for the leader of the free world, but not recommended for other Americans by USPSTF," the alliance said.
The group finished by stating that it believes FIT-DNA and CTC should be moved to a "recommended" status by USPSTF for people who are unable or unwilling to have a colonoscopy, and that USPSTF should re-evaluate its recommendations.