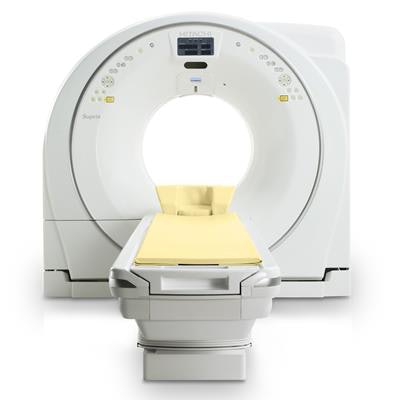
Hitachi Healthcare Americas has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Supria True64 compact CT system.
Supria True64 features a 40-mm detector and 64-slice imaging. It is XR-29 Smart Dose compliant and includes an "eco-mode" that reduces power consumption by up to 55% during idle periods, the company said.
 The Supria CT system from Hitachi.
The Supria CT system from Hitachi.