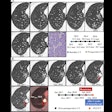
In a move that blazes a regulatory trail for developers of imaging artificial intelligence (AI) software, the U.S. Food and Drug Administration (FDA) has given its blessing to Viz.ai for Contact, an AI-based application that analyzes CT images and notifies providers that a patient might be having a stroke.
Importantly, the FDA's decision to grant marketing authorization for Viz.ai also establishes a new predicate device and regulatory classification, enabling subsequent computer-aided triage devices with the same intended medical imaging use to go through the 510(k) clearance process, according to the agency. The FDA's action follows a previous de novo decision in July 2017 that classified a breast imaging computer-aided diagnosis (CADx) software as a class II device and created a new generic device type, which was defined as "radiological computer-assisted diagnostic (CADx) software for lesions suspicious for cancer."
Both decisions were made by the FDA using its de novo premarket review pathway, which allows a vendor to petition the FDA to down-classify a novel device considered to have low to moderate risk and that had not previously been classified by the agency. Traditionally, these applications would by default be considered class III devices, which require the more-rigorous premarket approval (PMA) process.
The FDA noted that it's currently creating a regulatory framework for similar types of clinical decision-support applications that aim to assist providers in identifying the most appropriate treatment plan for a patient's disease or condition. The hope is to encourage developers to create, adapt, and expand the functionalities of the software to help providers diagnose and treat diseases and conditions, the FDA said.
In its decision on Viz.ai, the FDA reviewed data from a retrospective study of 300 CT images that assessed the independent performance of the image analysis algorithm and notification functionality against the performance of two trained neuroradiologists for detecting large-vessel blockages in the brain. The software, which is designed to analyze brain CT images and send text notifications to neurovascular specialists if it identifies a suspected large-vessel blockage, was shown in real-world evidence and the clinical study to demonstrate that the application could notify a neurovascular specialist sooner in cases where a blockage was suspected, according to the FDA.
"The algorithm will automatically notify the specialist during the same time the first-line provider is conducting a standard review of the images, potentially involving the specialist sooner than the usual standard of care in which patients wait for a radiologist to review CT images and notify a neurovascular specialist," the FDA said in a statement. "The notification can be sent to a mobile device, such as a smartphone or tablet, but the specialist still needs to review the images on a clinical workstation."
Viz.ai is intended to be used by neurovascular specialists such as vascular neurologists, neurointerventional specialists, or other professionals with similar training, and the FDA emphasized that it's limited to the analysis of imaging data and should not be used as a replacement of a full patient evaluation or solely relied upon to make or confirm a diagnosis.