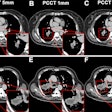
Software developer ClariPi has received clearance from the U.S. Food and Drug Administration (FDA) for its ClariCT.AI artificial intelligence-based CT denoising algorithm.
Software developer ClariPi has received clearance from the U.S. Food and Drug Administration for its ClariCT.AI artificial intelligence-based CT denoising algorithm.
First demonstrated at RSNA 2018, ClariCT.AI uses convolutional neural network technology to reduce noise and enhance image clarity in low- and ultralow-dose CT scans. The software was trained on over 1 million patient images.
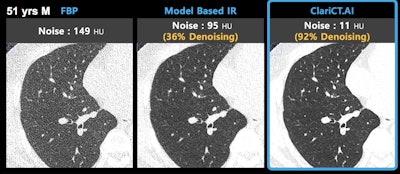
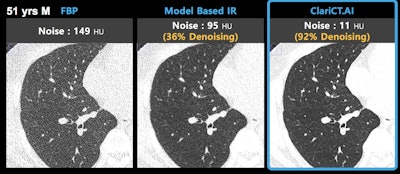 Image demonstrates ClariCT.AI algorithm applied to CT scan acquired at 120 kV and 3 mAs. Image courtesy of ClariPi.
Image demonstrates ClariCT.AI algorithm applied to CT scan acquired at 120 kV and 3 mAs. Image courtesy of ClariPi.The algorithm is based on Clarity Engine, which selectively separates image noise while enhancing underlying structures, thus providing images with restored clarity, according to the company. The software could improve the reading confidence of radiologists as well as the performance of computer-aided analysis software.
ClariPi will demonstrate the software at RSNA 2019. The software has also received the CE Mark in Europe.