Metabolic metrics tracked on PET/MRI and morphologic metrics tracked on CT show promise for evaluating tumor response to treatment and overall survival for patients with pancreatic ductal adenocarcinoma, according to a study published December 10 in the American Journal of Roetgenology.
The study results suggest that imaging metrics could help guide treatment for the disease, wrote a team led by Dr. Ananya Panda of the Mayo Clinic in Rochester, MN.
"Imaging metrics associated with pathologic response and overall survival in pancreatic ductal adenocarcinoma could help guide clinical management and outcomes for patients with [the disease] receiving emergent therapeutic interventions," the group wrote.
Neoadjuvant therapy is commonly used in patients with borderline-resectable or locally advanced pancreatic ductal adenocarcinoma, and tumor response to treatment is a "surrogate for systemic disease control and highly correlates with survival benefit," Panda and colleagues noted. Patients who don't respond to neoadjuvant therapy often experience disease relapse after surgery, making the benefit of surgery in patients without treatment response a debatable question.
That's why having biomarkers that could help predict treatment response prior to surgery could help clinicians guide drug therapy and reduce the chances of it being ineffective, select patients who could benefit from surgery, and time the surgery appropriately, according to the team.
So the investigators conducted a research study that included 44 patients with pancreatic cancer who underwent PET/MRI exams before and after treatment but before surgery between 2016 and 2019. The researchers compared metabolic metrics from the PET/MRI exams and morphologic metrics from the CT exam to patients' pathology scores. Metrics included the following:
- Complete metabolic response
- Change in maximum standardized uptake values (SUVmax)
- Change in glucose-corrected SUVmax
- Response Evaluation Criteria in Solid Tumours (RECIST) on CT
- Change in tumor volume on CT
Of the 44 patients, 43% had tumors that responded to treatment ("responders") and 57% had tumors that did not ("nonresponders"). Median overall survival was 24 months.
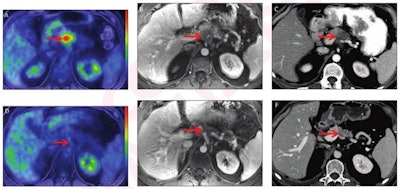 52-year-old man with locally advanced pancreatic adenocarcinoma and major pathologic response. Pretreatment (A-C) and post-treatment (D-F) images after eight cycles of systemic Folfirinox and consolidative chemoradiation. Baseline CA 19-9 was 145 U/ml. Pretreatment whole-body axial fused PET/MRI showed FDG-avid lesion in body of pancreas (arrow, A) with SUVmax 7.1 and SUVgluc 8.0. Lesion was hypoenhancing on axial contrast-enhanced T1-weighted MR image (arrow, B) from focused abdominal PET/MRI and on CT (arrow, C). Pretreatment CT tumor volume was 10.3 cm3. Post-treatment whole-body axial fused PET/MRI showed complete metabolic response (arrow D) with SUVmax 1.9 and SUVgluc 1.9. Lesion was indistinct on axial contrast-enhanced T1-weighted MRI (arrow, E) and CT (arrow, F), and there was upstream pancreatic parenchymal atrophy. Post-treatment CT tumor volume was 0.46 cm3. There was normalization of CA 19-9. Relative change in SUVmax was -73%, and relative change in SUVgluc was -76%. Based on change in tumor size, response was categorized as partial response per RECIST. Relative change in tumor volume was -96%. Pathology showed major pathologic response (College of American Pathologists score 1). Images and caption courtesy of the American Roentgen Ray Society and the American Journal of Roentgenology.
52-year-old man with locally advanced pancreatic adenocarcinoma and major pathologic response. Pretreatment (A-C) and post-treatment (D-F) images after eight cycles of systemic Folfirinox and consolidative chemoradiation. Baseline CA 19-9 was 145 U/ml. Pretreatment whole-body axial fused PET/MRI showed FDG-avid lesion in body of pancreas (arrow, A) with SUVmax 7.1 and SUVgluc 8.0. Lesion was hypoenhancing on axial contrast-enhanced T1-weighted MR image (arrow, B) from focused abdominal PET/MRI and on CT (arrow, C). Pretreatment CT tumor volume was 10.3 cm3. Post-treatment whole-body axial fused PET/MRI showed complete metabolic response (arrow D) with SUVmax 1.9 and SUVgluc 1.9. Lesion was indistinct on axial contrast-enhanced T1-weighted MRI (arrow, E) and CT (arrow, F), and there was upstream pancreatic parenchymal atrophy. Post-treatment CT tumor volume was 0.46 cm3. There was normalization of CA 19-9. Relative change in SUVmax was -73%, and relative change in SUVgluc was -76%. Based on change in tumor size, response was categorized as partial response per RECIST. Relative change in tumor volume was -96%. Pathology showed major pathologic response (College of American Pathologists score 1). Images and caption courtesy of the American Roentgen Ray Society and the American Journal of Roentgenology.Panda and colleagues found that pre- and post-treatment changes in tumor metrics on PET/MRI and CT were associated with pathologic response.
| Changes in metabolic metrics on PET/MRI and CT scans | ||
| Responders | Nonresponders | |
| Complete metabolic response | 90% | 40% |
| Change in SUVmax | -70% | -37% |
| Change in glucose-corrected SUVmax | -74% | -30% |
| RECIST response on CT | 93% | 50% |
| Change in tumor volume on CT | -85% | 57% |
Although the patient cohort for this study was small, the results suggest imaging has a key role to play in the assessment and management of pancreatic ductal adenocarcinoma, wrote Dr. Jean Lee of the University of Washington in Seattle in an accompanying editorial.
"This study opens the door in showing the feasibility of imaging as a prognostic marker," she wrote. "FDG-PET/MRI is not yet widely available but provides enormous possibility for radiologists and other nuclear medicine physicians to meaningfully impact the outcomes of patients with borderline resectable/locally advanced pancreatic cancer."