A photon-counting CT scanner from Siemens Healthineers has received clearance from the U.S. Food and Drug Administration (FDA), a move the FDA said was the "first major imaging device advancement" in CT in nearly a decade.
Naeotom Alpha uses the emerging technology of photon-counting CT, in which each individual x-ray photon is measured as it passes through the patient's body. This differs from existing CT instrumentation, in which the scanner's detectors measure the total energy in many x-rays at once.
"By 'counting' each individual x-ray photon, more detailed information about the patient can be obtained and used to create images with less information that is not useful in the review and analysis," the FDA said in a September 30 statement on the clearance.
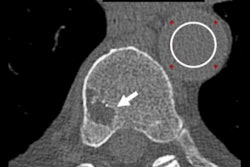
Proponents of photon-counting CT believe the scanners can be used to give radiologists much more detailed images at a lower radiation dose than conventional CT. Photon-counting images have a higher contrast-to-noise ratio, resulting in higher resolution and the correction of artifacts like beam hardening.
Research work into photon-counting CT has been underway at the Mayo Clinic in Rochester, MN, where investigators began working with a prototype scanner made by Siemens that could image humans in 2014. Mayo and Siemens have been working closely together on the project since the first human images were acquired in August 2015.
Mayo began using a second-generation photon-counting CT scanner in 2020, and a third generation of the technology went into operation in April 2021 as part of the collaboration between the two parties. In terms of importance, Mayo researchers have compared photon-counting CT with other major advances in CT instrumentation such as spiral CT, multidetector-row CT, and dual-energy CT.
Photon-counting CT produces "striking" images of the lungs, for example, according to Mayo researchers who reported their experiences with the system in 2019. The technology has inherently lower noise than conventional CT, and it can produce images with a spatial resolution of 0.15 mm without sacrificing radiation dose efficiency. Photon-counting CT also can perform simultaneous single-kV spectral imaging.
The FDA noted that Naeotom Alpha was cleared through the agency's 510(k) pathway, which is used for medical devices that are judged to be "substantially equivalent" to other products on the market.