N-acetylcysteine (NAC) is a promising compound for protecting cells from DNA damage caused by ionizing radiation in medical imaging, according to preclinical research presented Tuesday morning at RSNA 2021 in Chicago.
A group at the University of Utah's Center for Advanced Imaging identified NAC as a radioprotective agent in human peripheral blood mononuclear cells (PBMCs) and then tested the compound in an animal model. The team found that cells treated with NAC showed up to a 76% reduction in double-strand DNA breaks (DSBs), compared with untreated cells following ionizing radiation exposure.
"NAC significantly reduces DSBs in both human PBMCs and mouse model PBMCs when administered prior to irradiation," said interventional radiology fellow Dr. Tyler Smith.
Children and patients with genetic disorders that affect DNA repair are particularly vulnerable to cancers from DNA damage caused by ionizing radiation. For instance, patients with Li-Fraumeni syndrome (LFS) have a mutation in p53 that inhibits normal DNA repair. LFS patients are nearly 100% risk for cancer, Smith said.
The group tested eight compounds in cellular lab studies, including atorvastatin, resveratrol, and vitamin C, for radioprotective effects in PBMCs obtained from healthy volunteers and patients with LFS. In all screening tests, NAC was more effective in preventing radiation-induced DSBs. The cells were cultured in the presence of NAC at a concentration of 3 mg/mL, while a companion set of PBMCs were left untreated to serve as controls.
"NAC was very consistent for providing radioprotection and that's why we took it to the next step," Smith said.
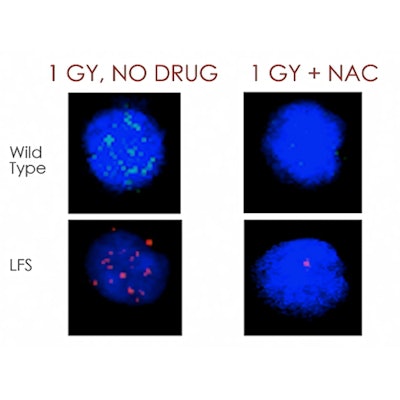
The researchers obtained a colony of wild-type P53 +/+ and heterozygous P53 +/- mice for the study. Groups of four mice each were treated with NAC and 1.0 Gy irradiation or no drug and 1.0 Gy of radiation. An hour after irradiation, the researchers collected blood from the mice and analyzed the PBMCs on confocal microscopy. The number of nuclear foci formed as a result of DSBs was counted per cell in the irradiated and untreated groups.
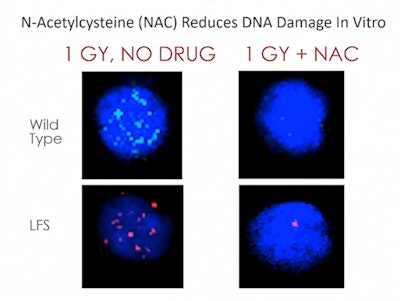 Image courtesy of Dr. Tyler Smith.
Image courtesy of Dr. Tyler Smith.Mice irradiated with 1.0 Gy had substantial increases in DSBs in PBMCs, the team found. In the untreated and unexposed baseline group, the number of foci identified per cell was 3.1 and 3.5. Following 1.0 Gy of radiation, the number of foci increased to 12.5 and 14.8.
In mice treated with 140 mg/kg of NAC and irradiated an hour later, the study authors found no significant changes in the number of foci per cell, compared with the untreated and unirradiated mice. The number of foci per cell in the NAC + 1.0 Gy group in both wild-type and heterozygous mice was substantially and significantly less than the number of foci in the group that had 1.0 Gy of radiation but no prior NAC treatment, the researchers found.
"Overall, we saw about a 60% to 75% reduction in double-stranded DNA breaks in our mouse models," Smith said.
NAC is a common agent in medication for asthma and cystic fibrosis and is used routinely in clinical practice. Could NAC supplementation have an effect on survival or treatment in vulnerable cancer patients?
Maybe, Smith said. First, the group plans future studies to assess the timing of administration for NAC, as well as the effective dose.
"If these results can be validated in humans, NAC supplementation could be developed as a method for reducing DSBs in patients due to ionizing radiation," Smith concluded.
The study was funded by RSNA's Research and Education Foundation.