Samsung Electronics subsidiary NeuroLogica has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its BodyTom 64 Point-of-Care Mobile CT scanner.
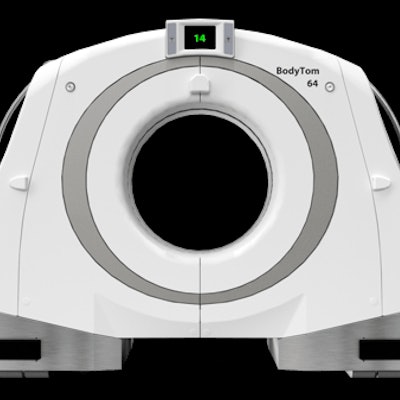
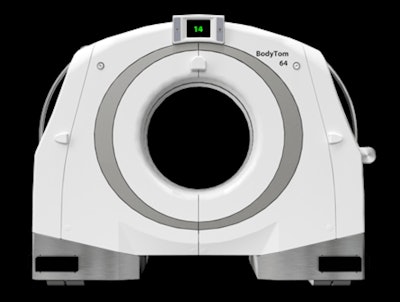 The NeuroLogica BodyTom 64 Point-of-Care Mobile CT scanner.
The NeuroLogica BodyTom 64 Point-of-Care Mobile CT scanner.The BodyTom 64 uses Linux as its operating system and can generate up to 64 cross-sectional CT images.
The scanner is designed for head-to-toe trauma imaging of emergency patients. It can also be used during neurosurgery and for extracranial procedures as well as for interventional radiology. Internal lead shielding and battery operation allow any standard trauma bay to be used as a CT imaging suite, according to NeuroLogica.
The BodyTom 64 can be combined with any radiolucent skull fixation device for neuroimaging during surgical procedures. In addition, it can help optimize workflows by remaining ready to rescan for each stage of needle guidance during interventional radiology procedures, the company added.