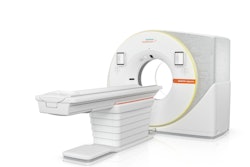
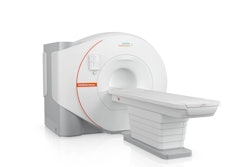
The U.S. Food and Drug Administration (FDA) has cleared Siemens Healthineers' Biograph Trinion PET/CT scanner.
The device features an air-cooled digital detector based on lutetium oxyorthosilicate (LSO) crystal elements which delivers high spatial resolution; a time-of-flight performance of 239 picoseconds for small lesion detectability; and sensitivity up to 128 cps/kBq, according to the firm. Biograph Trinion's platform allows for PET, CT, and postprocessing workflows on one system without the need for a separate CT exam or postprocessing, and postprocessing imaging applications include oncology, theranostics, cardiovascular imaging, and neurology imaging, Siemens said.