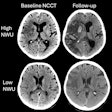
Bracco Diagnostics will halt development of three of its barium products but launch three new products over the next two years.
In a statement, the company said it is discontinuing its non-FDA-approved barium products due to market changes, evolving procedural changes, and a “significant decline” in product usage. Bracco’s planned product launches over the next 24 months include the following:
- CitraClear, a flavored beverage designed to enhance patient satisfaction during preparation for imaging procedures, in the third quarter of 2024.
- Varibar (barium sulfate) "mini" packaging, a new option for healthcare facilities for the U.S.-approved contrast agent, in the fourth quarter of 2024.
- A new flavor in its CT barium line in 2025.
The product portfolio update is part of the company's “rationalization process” to ensure that its resources are allocated effectively, noted Cosimo De Pinto, Bracco Diagnostics’ senior vice president of sales and marketing.