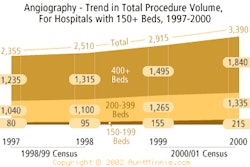
GE Medical Systems has received Food and Drug Administration 510(k) clearance for its Innova 4100 large-format flat-panel digital fluoroscopy system. Targeted for interventional radiology procedures, Innova 4100 employs the Waukesha, WI-based vendor's Revolution flat-panel detector and three-axis gantry positioning.
By AuntMinnie.com staff writers
November 27, 2002
Related Reading
GE, Deus to partner in lung CAD, November 26, 2002
GE adds to cardiac offerings, November 19, 2002
GE acquires micro-imaging firm, November 12, 2002
GE adds to PET offerings, October 28, 2002
GE inks deal with optical imaging firm, October 25, 2002
Copyright © 2002 AuntMinnie.com