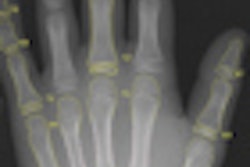
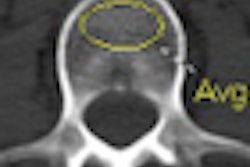
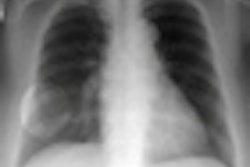
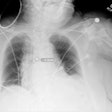
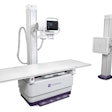
X-ray technology developer InfiMed has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Nexus-DRF multipurpose digital imaging system.
The latest addition to its i5 series, Nexus-DRF combines radiography/fluoroscopy (R/F), digital radiography (DR), and cardiac capabilities on one imaging platform using various flat-panel detectors, according to the vendor.
InfiMed is offering the unit as either a full system or as a software development kit.