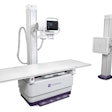
Siemens Healthcare has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Multix Fusion digital radiography (DR) system.
Now commercially available in the U.S., Multix Fusion can handle the full spectrum of routine radiography procedures and is suitable for institutions with smaller budgets, according to the vendor. It features a digital cassette-sized flat-panel detector with nearly unlimited positioning flexibility, a height-adjustable table with a 660-lb weight capacity, and a touchscreen at the overhead tube suspension to support exam parameter changes at the patient's bedside, Siemens said.
Multix Fusion includes Siemens' DiamondView Plus on-board postprocessing tool; automated image processing provides settings based on organ programs, Siemens said. In addition, a 71-inch telescopic column allows the tube to be pulled down directly above the floor, so patients do not need to climb onto the table for foot or other such exams, according to the company.
Several Multix Fusion variants are available, ranging from the simplest version with a ceiling-mounted tube and cassette to the complete digital x-ray system with the cassette-sized flat-panel detector, table, and wall stand. Siemens introduced the analog version of Multix Fusion in March.