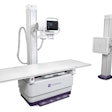
Shimadzu Medical Systems USA has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Sonialvision G4 universal digital radiography/fluoroscopy (R/F) system.
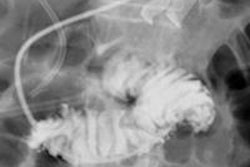
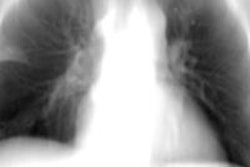
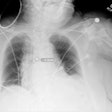
Featuring a 17 x 17-inch flat-panel detector with Shimadzu's next-generation digital imaging platform, Sonialvision G4 is suitable for a wide variety of examinations, including angiography, endoscopy, video fluoroscopy for gastrointestinal series and swallowing exams, orthopedic exams, and general radiography, Shimadzu said. It can accommodate all sizes of ambulatory and stretcher/wheelchair-confined patients and also includes workflow-efficiency features aimed at improving technologist productivity, according to the vendor.
The system yields a pixel pitch of 139 µm and has a movement range of 202.5 cm, enabling image capture of the entire body without moving the patient, Shimadzu said. It can easily switch between large and small fields-of-view, according to the firm. The vendor also noted the systems' real-time digital filtering processes that suppress halation near skin surfaces and around shadows where organs overlap.
Offering a lower dose profile that's well-suited for pediatrics, Shimadzu's slot-beam functionality is available as an advanced application option for long spine and limb imaging, according to the firm.
Shimadzu said that its dealers throughout the U.S. and in the Caribbean are now accepting orders for Sonialvision G4.