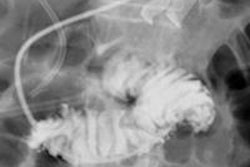
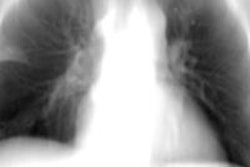
Siemens Healthcare has received clearance from the U.S. Food and Drug Administration (FDA) for its Artis One angiography system.
Artis One is designed for routine interventions, including revascularizations of peripheral vessel occlusions, functional tests of dialysis shunts in patients with kidney failure, diagnostic or minimally invasive angiographic treatment of narrowed coronary arteries, and pacemaker implantations, Siemens said.
Artis One also has ClearStent Live, an application for interventional cardiology procedures that freezes motion in the region of the stent. It also includes HeartSweep, which uses dual-axis rotational angiography to image the entire heart in a single C-arm movement instead of multiple acquisitions from different projections.