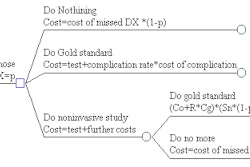
CryoVascular of Los Gatos, CA, has received U.S. Food and Drug Administration clearance to market its PolarCath peripheral transluminal angioplasty system. The device is used for treating clogged leg arteries via a liquid nitrous oxide-filled balloon that opens the vessel. The device debuted at the Transcatheter Cardiovascular Therapeutics symposium in Washington, DC, this week. It is expected to be commercially available in the first quarter of 2003.
By AuntMinnie.com staff writersSeptember 27, 2002
Copyright © 2002 AuntMinnie.com