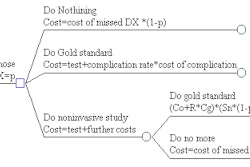
BioCure, an embolotherapy company spun off from the Novartis subsidiary CIBA Vision, has received Food and Drug Administration 510(k) marketing clearance for Bead Block, its polyvinyl alcohol (PVA) embolic microsphere product. The product will be used by interventional radiologists to treat hypervascular tumors and arteriovenous malformations, according to the Norcross, GA-based firm.
A license to the product has been granted to Biocompatibles International of Surrey, U.K., which expects to launch Bead Block in the coming months. The company also plans to pursue clinical trials to gain regulatory approval for a uterine fibroid embolization indication for the product.
By AuntMinnie.com staff writersJanuary 15, 2003
Copyright © 2003 AuntMinnie.com