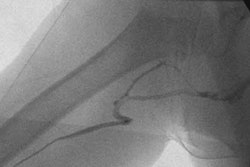
Interventional device developer Abbott Laboratories of Abbott Park, IL, said it has received conditional approval from the Food and Drug Administration for an investigational device exemption (IDE) application for the ZoMaxx drug-eluting stent.
The firm said it will begin enrolling the first 250 of an anticipated 1,670 patients from approximately 80 U.S. centers into its ZoMaxx II drug-eluting coronary stent clinical trial.
The trial will compare clinical outcomes in patients who are treated with the ZoMaxx drug-eluting coronary stent with patients who receive the Boston Scientific Taxus Express2 drug-eluting stent, Abbot said. The primary end point of the trial is nine-month ischemia-driven target-vessel revascularization, according to the vendor.
By AuntMinnie.com staff writers
April 7, 2005
Related Reading
Abbott to launch carotid stent trial, January 20, 2005
Abbott plans drug-eluting stent trial, September 28, 2004
Abbott starts ZoMaxx stent trial, September 17, 2004
Abbott debuts StarClose, May 26, 2004
Abbott launches Perclose ProGlide, May 13, 2004
Copyright © 2005 AuntMinnie.com