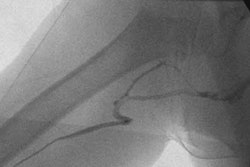
Interventional device vendor Guidant has received investigational device exemption (IDE) conditional approval from the Food and Drug Administration to begin the U.S. portion of its Spirit III clinical trial.
Spirit III is a large-scale clinical trial evaluating the safety and efficacy of Guidant's drug-eluting stent system for the treatment of coronary artery disease, according to the Indianapolis-based firm. The randomized, single-blind trial compares Guidant's Xience V everolimus-eluting coronary stent system, using Guidant's cobalt chromium Multi-Link Vision coronary stent system platform, versus the firm's Taxus Express2 paclitaxel-eluting coronary stent system.
By AuntMinnie.com staff writers
May 5, 2005
Related Reading
Guidant shareholders OK acquisition, April 27, 2005
Guidant reports Q1 revenue growth, April 21, 2005
Verdicts come down in Medtronic/Guidant dispute, February 21, 2005
GE, Guidant offer free screenings, February 3, 2005
Guidant reports record year, January 27, 2005
Copyright © 2005 AuntMinnie.com