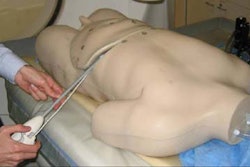
Oncology device developer RITA Medical Systems has delayed U.S. shipments of its Habib4X resection device.
The product coagulates a surgical resection plane to facilitate a fast dissection with limited blood loss. The Fremont, CA-based firm said it has received isolated reports that the sterile packaging of some products had been compromised during shipping.
RITA said it has rejected subsequent product shipments from the manufacturer, and has requested that all products previously shipped to U.S. customers be returned for replacement. The firm said it will not resume shipment of Habib4X products to the U.S. market until a new packaging design is implemented and validated by the manufacturer, and approved by RITA.
By AuntMinnie.com staff writers
September 27, 2005
Related Reading
RITA gets 510(k), August 19, 2005
Martinez joins RITA, August 18, 2005
RITA launches safety needle, August 16, 2005
RITA debuts new ports, July 28, 2005
RITA brings new auditor on board, July 5, 2005
Copyright © 2005 AuntMinnie.com