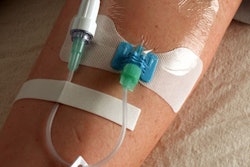
Interventional technology firm Cook reported that the U.S. Food and Drug Administration has cleared the latest iteration of the developer's Spectrum catheter.
The five-lumen central venous catheter is designed to both improve multiple treatment administration and provide increased protection against hospital-based catheter-related bloodstream infections, according to Bloomington, IN-based Cook.
The device is available now, the company said.
By AuntMinnie.com staff writers
July 25, 2006
Related Reading
Cook nets FDA nod, July 6, 2006
Cook Med Institute expands, June 29, 2006
Cook launches MRI-safe embolization coil, March 31, 2006
Cook reports success with retrievable vena cava filter, December 6, 2006
Cook unveils new angioplasty balloon catheter, December 1, 2005
Copyright © 2006 AuntMinnie.com