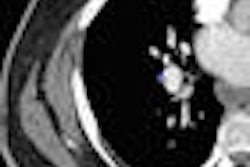
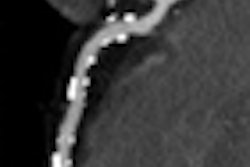
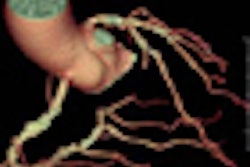
Abbott Laboratories has begun a U.S. clinical trial for its Absolute Pro peripheral stent.
The study will evaluate the safety and efficacy of Absolute Pro, which is designed for treatment of iliac artery disease. The trial, called Mobility, is designed to study 150 patients at up to 50 centers in the U.S., Abbott said.
The first patient was enrolled at Charleston Area Medical Center in Charleston, WV, according to Abbott of Abbott Park, IL. The primary end point will be a composite measure of major adverse events at nine months, including death due to any cause, heart attack, clinically driven target lesion revascularization, and amputation on the treated side.
Related Reading
Abbott gets FDA nod for Xience V, July 3, 2008
U.S. panel backs Abbott's drug-coated heart stent, December 3, 2007
Clots low with Abbott stent for 2 yrs: FDA staff, November 28, 2007
Abbott begins stent trial for cardiovascular disease, July 20, 2007
GE, Abbott break off acquisition, July 11, 2007
Copyright © 2009 AuntMinnie.com