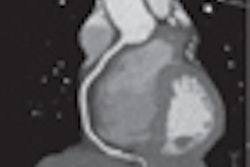
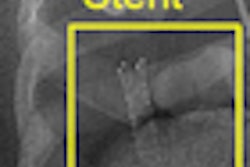
Coronary imaging developer InfraReDx has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its LipiScan intravascular ultrasound (IVUS) coronary imaging system.
LipiScan IVUS is a cardiac catheter that combines intravascular ultrasound and near-infrared spectroscopy to help cardiologists identify and characterize plaques that complicate stenting and are associated with acute coronary events, according to the Burlington, MA-based vendor.
InfraReDx expects to launch the system in the U.S. by the end of 2010; European regulatory approval and launch is anticipated during 2011.
Related Reading
InfraReDx announces first clinical use of LipiScan, May 16, 2008
FDA clears InfraReDx's LipiScan, April 30, 2008
InfraReDx receives FDA clearance, October 19, 2006
Near-infrared spectroscopy can identify vulnerable plaques in atherosclerosis, September 29, 2002
Copyright © 2010 AuntMinnie.com