Healthcare technology firm Covidien said that the U.S. Food and Drug Administration (FDA) has approved the premarket approval (PMA) application for its Pipeline embolization device.
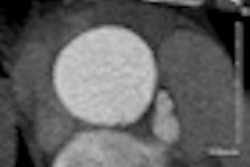
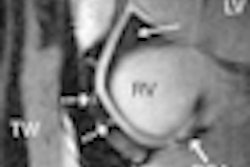
Pipeline is indicated for the endovascular treatment of adults with large or giant wide-necked intracranial aneurysms in the internal carotid artery from the petrous to the superior hypophyseal segments, according to the firm.
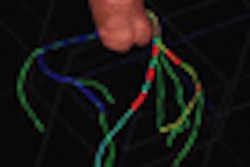
The embolization device is designed to divert blood flow away from the aneurysm to provide a complete and durable aneurysm embolization while maintaining patency of the parent vessel, Covidien said. It will be available at existing U.S. clinical sites beginning this quarter.