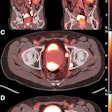
United Imaging Healthcare has received U.S. Food and Drug Administration (FDA) clearance for a new PET/MRI scanner that can scan the entire body in 20 minutes.
The new scanner, uPMR 790, is a high-definition time-of-flight system that represents United's effort to balance patient comfort with high-quality imaging, according to the company. The system is based on digital silicon photomultipliers (SiPMs) with lutetium-yttrium oxyorthosilicate (LYSO) scintillation crystals. These specifications provide 2.8-mm spatial resolution under National Electrical Manufacturers Association (NEMA) criteria and a 32-cm axial field of view.
uPMR 790 can also be used with compressed sensing MRI technology for whole-body 3D isotropic MR imaging, which gives clinicians high isotropic spatial resolution for visualizing small lesions while speeding up acquisition times to maximize patient comfort, according to United.
The platform also supports research with United's uSync Research platform, which enables research in areas such as simultaneous tracking of PET and MRI tracers, real-time cardiac PET/MR, functional neurological PET/MR, and multiparametric radiometrics.