Proponents of a bill pending in the U.S. Congress that would ensure adequate reimbursement for diagnostic radiopharmaceuticals for use with PET to diagnose Alzheimer's disease and other neurodegenerative conditions took their cause to the nation's capital on January 29.
Among the provisions in the Medicare Diagnostic Radiopharmaceutical Payment Equity Act of 2019 (HR 3772) is fair reimbursement for a number of PET radiotracers.
"As we try to push Alzheimer's diagnosis earlier and earlier in the disease process, we want to diagnose people before they have any symptoms and certainly by their first symptom," said Dr. William Klunk, PhD, distinguished professor of psychiatry and advisory co-director at the Alzheimer's Disease Research Center at the University of Pittsburgh. By determining which potential, future Alzheimer's patients are amyloid-positive, PET "could become a tool to make life easier for these people when they seek more information about their condition."
Currently, the U.S. Centers for Medicare and Medicaid Services classifies radiopharmaceuticals, including those used for amyloid PET imaging, relative to the cost of a procedure in hospital outpatient settings. This approach discourages the use of many radiopharmaceuticals in the Medicare hospital outpatient setting, which leads to limited patient access and stifles research and innovation, proponents contend.
At least one study solidly endorsed the efficacy of amyloid PET scans to confirm a diagnosis of Alzheimer's disease. The April 2019 study by researchers from the University of California, San Francisco determined that amyloid PET changed diagnoses to or from Alzheimer's for 35% of patients and consequently changed their clinical management in more than 60% of the cases. Their findings also indicated that amyloid PET scans could influence how Alzheimer's patients are treated as soon as 90 days after imaging.
The study included three beta-amyloid PET tracers, all of which are approved for clinical use by the U.S. Food and Drug Administration (FDA) for the diagnosis of Alzheimer's disease and are included in the pending legislation for reimbursement:
- Florbetapir (Amyvid, Avid Radiopharmaceuticals)
- Flutemetamol (Vizamyl, GE Healthcare)
- Florbetaben (Neuraceq, Life Molecular Imaging)
To safeguard against overutilization or unnecessary amyloid PET imaging, Klunk advocated following current appropriate use criteria guidelines to determine which patients should undergo these types of scans.
"There is no need to fear unregulated use [of amyloid PET imaging] for everyone who can't find their keys or glasses," he told the briefing's attendees. "We want to have appropriate use guidelines. A person should be objectively confirmed by a comprehensive evaluation by an expert that the scan is expected to increase the diagnostic certainty."
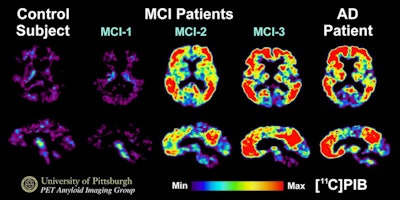 PET images were performed with carbon-11 labeled Pittsburgh Compound B (C-11 PiB) on normal control subjects (far left), three different patients with mild cognitive impairment (MCI) (center images) and a patient with mild Alzheimer's disease (far right). Some patients with MCI have amyloid levels similar to control subjects, while some amyloid levels in MCI patients resemble Alzheimer's conditions. Images courtesy of Dr. William Klunk, PhD, and the University of Pittsburgh.
PET images were performed with carbon-11 labeled Pittsburgh Compound B (C-11 PiB) on normal control subjects (far left), three different patients with mild cognitive impairment (MCI) (center images) and a patient with mild Alzheimer's disease (far right). Some patients with MCI have amyloid levels similar to control subjects, while some amyloid levels in MCI patients resemble Alzheimer's conditions. Images courtesy of Dr. William Klunk, PhD, and the University of Pittsburgh.The Congressional briefing included comments from Alzheimer's patient Geri Taylor, who told the gathering that only when her amyloid PET scan results were positive did she definitively know she had the disease. She and her husband, Jim, now campaign to increase Alzheimer's clinical trial participation, which is dependent on access to amyloid PET imaging.
The congressional briefing was sponsored by the Medical Imaging and Technology Alliance (MITA), the Council on Radionuclides and Radiopharmaceuticals (CORAR), and the Society of Nuclear Medicine and Molecular Imaging (SNMMI).




















