The Society of Nuclear Medicine and Molecular Imaging (SNMMI) is throwing its support behind legislation introduced in the U.S. Congress that would change existing reimbursement policy toward PET radiopharmaceuticals.
The Facilitating Innovative Nuclear Diagnostics (FIND) Act of 2021 was introduced in Congress July 16 and received support from the SNMMI as well as its lobbying coalition of partners.
The bill, formerly called HR 3772, aims for a legislative fix to policy by the U.S. Centers for Medicare and Medicaid Services (CMS) of bundling the use of diagnostic radiopharmaceuticals in hospital outpatient procedures.
The SNMMI and its coalition of patient advocacy partners, including the Medical Imaging & Technology Alliance and the Council on Radionuclides and Radiopharmaceuticals, applauded the introduction.
"Innovative radiopharmaceuticals are revolutionizing the diagnosis and treatment of a wide variety of diseases, but under current CMS payment policies, these remarkable agents often are not available to Medicare beneficiaries, resulting in inequities in healthcare," said Dr. Richard Wahl, president of SNMMI, in a statement.
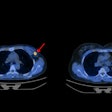
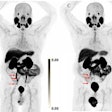
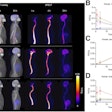
If passed, the FIND Act would provide patients greater access to a wide range of diagnostic radiopharmaceuticals for detecting conditions such as heart disease, Alzheimer's and Parkinson's disease, breast and prostate cancer, and neuroendocrine tumors. This legislation would also help providers better manage costs while delivering more targeted and cost-efficient care, according to the SNMMI.
The bill was introduced by U.S. Representatives Scott Peters (D-CA), Bobby Rush (D-IL), Neal Dunn (R-FL), and Greg Murphy (R-NC).