CereMark Pharma has launched a phase III trial of its PET imaging agent F-18 flornaptitril at Endeavor Health’s Neurosciences Institute in Evanston, IL.
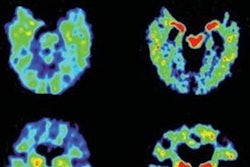
F-18 flornaptitril is a PET radiotracer with a mechanism of action that allows the detection of both brain tau aggregates and beta-amyloid plaques simultaneously within a single scan. It is being studied for effectiveness in the management of neurodegenerative diseases, including the ability to predict development and progression of Alzheimer’s disease and chronic traumatic encephalopathy.
 F-18 Flornaptitril binds with both tau aggregates and beta-amyloid plaque to enhance PET scan imaging of these pathological proteins in the brain.CereMark
F-18 Flornaptitril binds with both tau aggregates and beta-amyloid plaque to enhance PET scan imaging of these pathological proteins in the brain.CereMark
The tracer has already been tested safely in clinical investigational studies involving more than 550 patients with traumatic brain injuries or other neurological diseases, the company noted. Additional clinical sites for the trial will be announced in the coming months, CereMark added.