CereMark Pharma, a radiopharmaceutical company focused on neurodegenerative diseases, is planning a phase III clinical trial later this year to investigate the novel PET imaging agent F-18 flornaptitril.
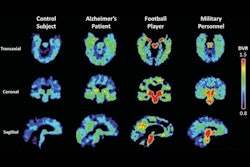
F-18 flornaptitril is intended to target two pathological proteins that occur in the development of Alzheimer’s disease and chronic traumatic encephalopathy. The investigational trial will focus on athletes and soldiers presenting with the initial indicators of mild cognitive impairment. CereMark recently announced a collaboration with Hall of Fame Health to support its upcoming clinical studies.
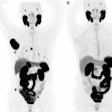
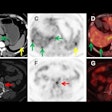
Understanding the regional distribution and density of beta-amyloid plaques and tau protein aggregates in brain regions is critical to understanding neurodegeneration progression and effective patient management, according to CereMark.
A recent decision to classify traumatic brain injuries as a chronic condition is a win for patients, while unbundling payments for PET radiotracers will drive growth and innovation within companies like CereMark, the company told AuntMinnie.com.