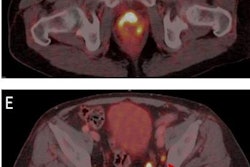
Bracco molecular imaging subsidiary Blue Earth Diagnostics has begun a second phase III clinical trial of its investigational radiohybrid PET imaging agent for prostate cancer.
The Spotlight clinical trial is a multicenter, single-arm study in the U.S. and Europe to evaluate the safety and diagnostic performance of rhPSMA-7.3 (F-18), Blue Earth's investigational prostate-specific membrane antigen-targeted radiohybrid PET imaging agent. Its primary goal is to evaluate the correct detection rate and positive predictive value of PET imaging with the agent compared with histopathology and confirmatory imaging results.
The trial will focus on men with suspected prostate cancer recurrence based on elevated prostate-specific antigen levels after prior cancer treatment. Thus far, patients have received doses of rhPSMA-7.3 at sites in Georgia, California, Texas, Virginia, and Finland.
Blue Earth is also conducting a second, ongoing phase III trial of the PET agent called the Lighthouse trial. The Lighthouse trial commenced in March and is investigating the agent's safety and diagnostic performance in men with newly diagnosed prostate cancer.