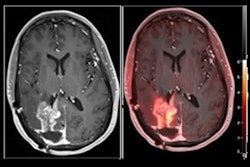
Bracco molecular imaging subsidiary Blue Earth Diagnostics announced that the first patient has been dosed in a phase III clinical study examining the use of its Axumin (fluciclovine fluorine-18, F-18) PET radiopharmaceutical for detecting recurrent brain metastases.
Currently approved for use in imaging recurrent prostate cancer, Blue Earth is pursuing additional applications for the radiotracer, such as detecting brain metastases. This application is being investigated in the REVELATE study, a phase III multicenter single-arm imaging study being conducted in the U.S. to assess the performance of PET with fluciclovine for detecting recurrent brain metastases in patients who have been treated with radiation therapy.
Blue Earth said the first patient in the REVELATE study has been dosed at Yale University under the auspices of Dr. Mariam Aboian, PhD.