A widely available amino acid PET radiotracer shows promise in helping clinicians distinguish between pseudoprogression and actual tumor progression in patients with brain tumors, according to a study published December 22 in the Journal of Nuclear Medicine.
In a small clinical trial, researchers at the University of Pennsylvania investigated the value of F-18 fluciclovine (Axumin, Blue Earth Diagnostics) PET for differentiating pseudoprogression from tumor progression in a group of patients with suspected recurrence of glioblastomas. The tracer performed well, with the study a first step in determining whether it could be used in routine clinical assessment of these patients, the authors wrote.
"Given the wide availability of F-18 fluciclovine in the U.S. as an [U.S. Food and Drug Administration] approved radiotracer, this study has immediate translational relevance," wrote corresponding author Dr. Ali Nabavizadeh, a radiologist at the university's Perelman School of Medicine.
The U.S. Food and Drug Administration (FDA) approved Axumin in 2016 for PET imaging in men who are suspected of having recurrent prostate cancer based on elevated prostate-specific antigen levels that occur after primary treatment. The radiotracer contains a fluciclovine amino acid labeled with an F-18 radioisotope that reveals increased cellular activity in glial cells in tumors. It is currently being investigated for other potential clinical uses.
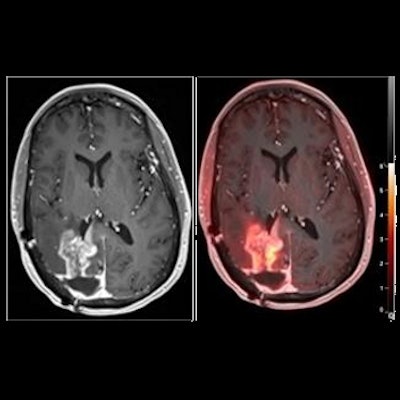
In this study, the researchers investigated its performance differentiating between tumor progression and pseudoprogression in glioblastoma, a type of brain tumor that remains incurable in patients following standard chemoradiotherapy. Although MRI is the current standard for tumor monitoring, it lacks specificity for detecting neoplastic progression in the brain, according to the authors.
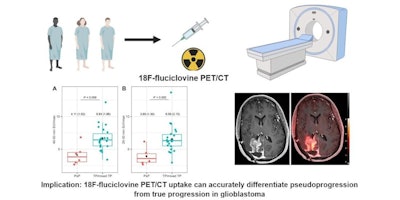 Graphical abstract. Image courtesy of the Journal of Nuclear Medicine
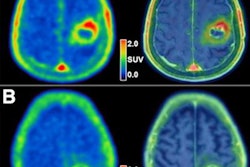
Graphical abstract. Image courtesy of the Journal of Nuclear MedicineThe researchers enrolled 30 glioblastoma patients with radiographic progression after first-line chemoradiotherapy who were planned for surgery. Patients underwent preoperative F-18 fluciclovine PET and MRI, with differences in PET radiotracer uptake between the types of tumors compared to results confirmed by biopsy lab tests.
Eighteen patients had tumor progression identified, four had mixed tumor progression, and eight patients had pseudoprogression. Patients with progression and mixed progression had significantly higher maximum standard uptake values (SUVmax) compared with patients with pseudoprogression (6.64 vs. 4.11, p = 0.009).
In addition, in patients who underwent imaging 40 to 50 minutes after radiotracer injection, an SUVmax cutoff of 4.66 provided a 90% sensitivity and 83% specificity for differentiating between tumor progression and pseudoprogression, according to the findings.
Overall, the results suggest that F-18 fluciclovine PET imaging can accurately differentiate pseudoprogression from tumor progression in glioblastoma patients, the authors stated.
"Larger, multicenter studies are warranted to determine whether amino acid PET with F-18 fluciclovine should be used in the routine assessment of post-treatment glioblastoma," Nabavizadeh and colleagues concluded.
Research funding was provided by Blue Earth for the study.