A new lutetium-177 (Lu-177)-based radiopharmaceutical therapy prolonged overall survival in patients with pleural metastases who were previously treated with standard therapies, according to a study published January 22 in the Journal of Nuclear Medicine.
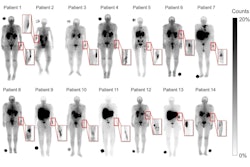
In a small number of patients with fibroblast activation protein (FAP)-positive pleural metastases, treatment with Lu-177 FAP-2286 achieved a disease control rate of 59%, with gallium-68 (Ga-68) FAP-2286 PET/CT imaging confirming the results, noted lead author Linwei Li, MD, of the Affiliated Hospital of Southwest Medical University in Luzhou, China, and colleagues.
“This study provides real-world evidence supporting FAP-targeted radiopharmaceutical therapy as a treatment option for this patient population and suggests FAP-based imaging is useful for response monitoring,” the group wrote.
FAP is highly expressed on cancer-associated fibroblast cells in over 90% of epithelial tumors and has emerged as a novel therapeutic target for cancer treatment, the authors explained. Recently, the group conducted a head-to-head study demonstrating that Ga-68 FAP-2286 PET/CT is more accurate in diagnosing pleural metastases than standard F-18 FDG PET/CT.
Furthermore, they noted that their center has accumulated preliminary evidence that Lu-177 FAP-2286 can treat various types of advanced tumors, such as lung cancer, rhabdoid meningioma, and bladder cancer. In this study, the group explored further use of the theranostic pair in patients with pleural metastases.
The researchers analyzed data from 17 patients with FAP-positive pleural metastases confirmed on baseline F-18 FDG PET/CT and Ga-68 FAP-2286 PET/CT scans. Patients received a median of two cycles of Lu-177 FAP-2286. Previously, they had been heavily treated with standard therapies, such as chemotherapy, immunotherapy, and radiation therapy.
The team assessed patient responses 24 hours after administering the treatment using Ga-68 FAP-2286 PET/CT, CT, and qualitative SPECT/CT, then calculated overall survival (OS) and progression-free survival, and noted any adverse events.
According to the results, the disease control rate (nonprogressive disease) was 59% (n = 10). The median OS among the group was 17.3 months, and the median progression-free survival was 4.1 months, the group reported. Patients who achieved disease control had significantly longer OS (20.3 months) compared with those with progressive disease (6.4 months, p = 0.004).
In addition, the treatment was well-tolerated, with no grade 3 or 4 adverse events reported. The most common adverse events were manageable grade 1 and 2 hematologic toxicities, according to the findings.
“To our knowledge, this is the first study to explore a potential new indication for FAP peptide-targeted radionuclide therapy and to compare FAP-based imaging for response monitoring,” the researchers wrote.
These results provide preliminary real-world clinical evidence that could inform future research, the researchers concluded.
The full study can be found here.