PET imaging with a copper-64 (Cu-64)-labeled nanoparticle appears safe in humans and warrants further development for detecting cancer and sarcoidosis, researchers in Boston have reported.
The finding is from a phase I human trial that determined the biodistribution and dosimetry of a novel radiotracer called Cu-64 Macrin, noted lead author Aileen O’Shea, MD, of Massachusetts General Hospital in Boston, and colleagues.
“We report the imaging findings, safety, and dosimetry of a macrophage-avid nanoparticle PET sensor (Cu-64 Macrin) in healthy human volunteers and a small cohort of patients with inflammatory disease. No safety events were observed after injection,” the group wrote. The study was published December 30 in the Journal of Nuclear Medicine.
Macrophages are innate immune cells that play a critical role in responses to infection and injury, yet the density of these cells in tissues has also been well correlated with a broad swath of diseases ranging from cancer to neurologic disorders, the authors explained. Given their importance, a molecular imaging approach to quantify their amounts in different tissues is highly desirable, they added.
To that end, the researchers developed Cu-64 Macrin, a large 17-nanometer tracer. In previous animal studies, they showed that the tracer accumulates virtually exclusively in macrophages. To evaluate it in humans, the team recruited five healthy participants who underwent tracer injections and then whole-body PET/MRI scans.
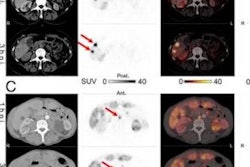
Additionally, as proof of principle, three patients with cancer (n = 1) or sarcoidosis (n = 2) underwent whole-body imaging and dedicated imaging of the chest or abdomen and pelvis.
According to the findings, no adverse or clinically detectable pharmacologic effects were observed in any of the participants at least one week after the study, establishing the safety of the imaging agent. Most of Cu-64 Macrin was cleared from the blood of participants after 1.3 hours, the researchers noted.
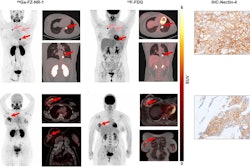
 Cu-64 Macrin uptake in sarcoidosis patient. Coronal fused F-18 FDG-PET/CT scan (A) shows minimal uptake in paratracheal lymph nodes (F-18 FDG SUVmax, 2.2; hepatic SUVmean, 3.3). Coronal fused PET/MR at same level (B) reveals Macrin efficiently labeled paratracheal and hilar lymph nodes (arrows) (SUVmax, 6.6; hepatic SUVmean, 17) with biopsy-confirmed active inflammation.Journal of Nuclear Medicine
Cu-64 Macrin uptake in sarcoidosis patient. Coronal fused F-18 FDG-PET/CT scan (A) shows minimal uptake in paratracheal lymph nodes (F-18 FDG SUVmax, 2.2; hepatic SUVmean, 3.3). Coronal fused PET/MR at same level (B) reveals Macrin efficiently labeled paratracheal and hilar lymph nodes (arrows) (SUVmax, 6.6; hepatic SUVmean, 17) with biopsy-confirmed active inflammation.Journal of Nuclear Medicine
“This first-in-human study demonstrates that Cu-64 Macrin, a novel macrophage-targeting PET tracer, has favorable safety and dosimetric profiles, supporting its further clinical development for imaging and detection of treatment responses in macrophage-mediated inflammatory, malignant, and cardiovascular diseases,” the group wrote.
The study sets the stage for larger prospective clinical trials in specific patient cohorts, with the goal being to determine whether the noninvasive technique can quantify dynamic changes in macrophage density at multiple sites over time, the researchers concluded.
The full study can be found here.