HONOLULU -- An AI-based framework can detect ischemic heart disease on myocardial perfusion MRI with only a very low dose of a gadolinium-based contrast agent, according to research presented at the annual meeting of the International Society for Magnetic Resonance in Medicine (ISMRM).
“By reducing the contrast dose, our approach holds promise for enhancing patient safety, especially in clinical settings that require serial imaging,” said presenter Khalid Youssef, PhD, of Indiana University School of Medicine.
The use of stress first-pass perfusion (FPP) on myocardial MRI to detect myocardial ischemia is a cornerstone in the diagnosis and management of ischemic heart disease. Gadolinium-based contrast agents (GBCAs) are needed to quantify myocardial blood flow, but come with the risk of brain deposition and potential cumulative effects. It’s also infeasible for serial FPP studies, according to Youssef.
Although reducing gadolinium doses is highly desirable for patient safety, lower doses diminish image quality and increase the risk for inaccurate detection, the authors noted. Khaled Youssef, PhD, of Indiana University School of Medicine
Khaled Youssef, PhD, of Indiana University School of Medicine
In their study, Youssef and colleagues from Indiana University, Purdue University, Harvard University, and the University of Calgary sought to determine if AI could enable an order-of-magnitude reduction in gadolinium contrast dose but still accurately detect myocardial ischemia.
They developed a multistage AI-powered imaging analysis/quantification pipeline that performs localization and segmentation, myocardial blood flow (MBF) quantification, and classification. In the first stage, convolutional neural networks detect and segment the myocardium and ventricles on the myocardial perfusion MR images.
Next, an algorithm extracts myocardial time curves and arterial input function in a patch-wise manner from the segmented regions. Myocardial blood flow is then quantified for each myocardial pixel with a multistage neural network, according to the researchers.
In testing on 20 patients with images acquired at 80% lower gadolinium dose, the method yielded MBF values on images that correlated highly with full-dose results, and outperformed Fermi-constrained deconvolution method (r² = 0.93 vs. r² = 0.68, p < 0.001). What’s more, it still produced strong results even at 88% lower dose and poor contrast-to-noise, again beating the Fermi method (r² = 0.86 vs. r² = 0.63, p < 0.001).
In further testing on 30 patients with a 90% dose reduction, the AI framework also achieved an area under the curve (AUC) for ischemia detection of 0.87, compared with 0.77 for the Fermi method. That difference was also statistically significant (p < 0.05).
“Our results indicate that the proposed deep-learning framework may achieve a high diagnostic performance for detection of stress-induced ischemia,” Youssef said.
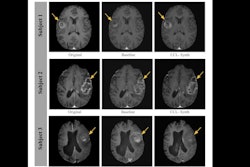
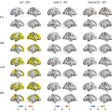
 Representative stress perfusion CMR case with 80% contrast dose reduction. Pixel-wise MBF maps and AHA 16-segment bull's eye plots are shown for full-dose and reduced-dose acquisitions. When analyzed using the proposed MST deep learning method, the reduced-dose data closely matches the full-dose data across basal, mid, and apical slices. In contrast, Fermi-deconvolution shows good agreement in apical and mid slices but overestimates MBF in some basal segments, indicating reduced accuracy under low-dose conditions. Image courtesy of the ISMRM.
Representative stress perfusion CMR case with 80% contrast dose reduction. Pixel-wise MBF maps and AHA 16-segment bull's eye plots are shown for full-dose and reduced-dose acquisitions. When analyzed using the proposed MST deep learning method, the reduced-dose data closely matches the full-dose data across basal, mid, and apical slices. In contrast, Fermi-deconvolution shows good agreement in apical and mid slices but overestimates MBF in some basal segments, indicating reduced accuracy under low-dose conditions. Image courtesy of the ISMRM.
The researchers now plan to validate their results in larger, multicenter studies.
Check out AuntMinnie’s full coverage of ISMRM 2025 here.