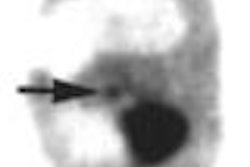
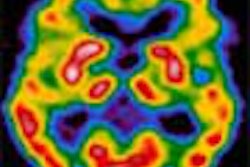
North American Scientific subsidiary Theseus Imaging has begun clinical enrollment and has imaged the first subject in a phase II/III clinical study of the use of Apomate for the detection of early response to chemotherapy in patients with non-small cell lung cancer.
The study is designed to enroll up to 140 patients in the U.S. and Europe with advanced non-small cell lung cancer, in order to assess the ability of Apomate for in vivo imaging of apoptosis and necrosis, two common forms of cell death. Apomate is a technetium-based radiopharmaceutical under development at the Chatsworth, CA, company.
It may take weeks or even months of treatment before any change in tumor size suggesting response to treatment can be detected by conventional imaging. Meanwhile, molecular imaging of cancer cell death with Apomate may detect response to treatment within one or two days of the initial dose of chemotherapy, according to the company.
Initial phase I data suggested that those subjects who showed increased Apomate uptake in their tumors within days of their first treatment had a statistically significant increase in tumor response to therapy and in survival time when compared to the group that did not.
Apomate is also in clinical trials to assess the extent of damaged myocardium following a heart attack, or following treatment of a heart attack, and to noninvasively evaluate heart transplant rejection.
By AuntMinnie.com staff writers
November 30, 2001
Related Reading
NAS revenues climb in Q3, August 29, 2001
NAS names Robert Morgan as VP, June 19, 2001
NAS taps Jones for Theseus VP post , April 10, 2001
Theseus starts Apomate phase II trials, April 3, 2001
North American Scientific dips into the red, March 1, 2001
Copyright © 2001 AuntMinnie.com